By the Doctor Philippe Kestemont
For “MEDITI”
 Our clinical research team (MEDITI) took part in the “Abo- BoNT-A 189 and 214” clinical studies into the liquid form of AboBoNT-A with the IPSEN laboratory. We are part of the global board for the launch of this new formulation, called ALLUZIENCE, from GALDERMA laboratories. Let us clarify some information about this “ready to use” neurotoxin.
Our clinical research team (MEDITI) took part in the “Abo- BoNT-A 189 and 214” clinical studies into the liquid form of AboBoNT-A with the IPSEN laboratory. We are part of the global board for the launch of this new formulation, called ALLUZIENCE, from GALDERMA laboratories. Let us clarify some information about this “ready to use” neurotoxin.
1. Why propose this formulation?
Doctors that use lyophilised type A neurotoxins on a daily basis are faced with various constraints and side effects:
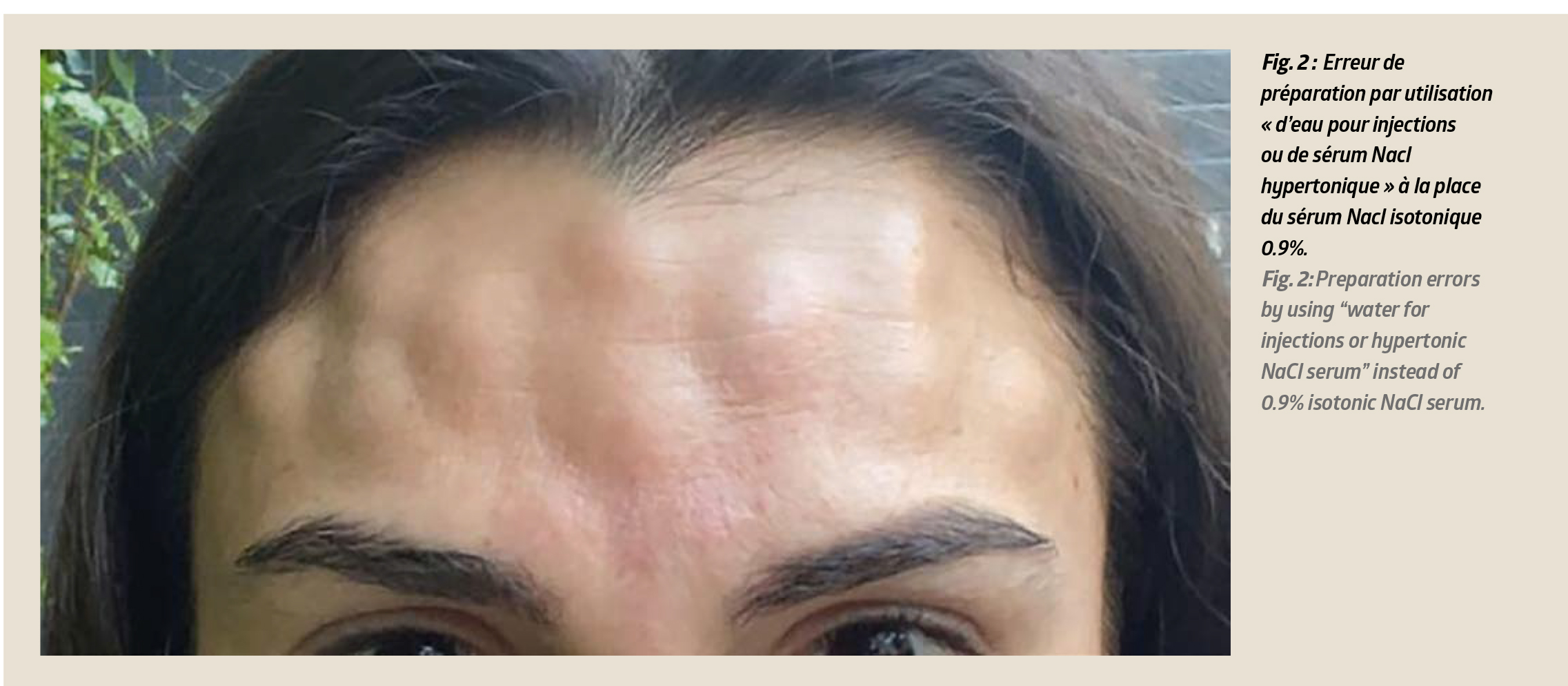
• Preparation errors by using “water for injections or hypertonic NaCl serum” instead of 0.9% isotonic NaCl serum. These handling errors lead to injection hyperalgesia and sometimes spectacular facial oedemas that require the injections to be halted immediately and the bottle to be disposed of (Fig. 2).
• Variable clinical results due to individual variations in the volume of serum used for dilution: 0.63cc, 0.70cc, 1cc, 1.25cc, etc.
• Septic problems due to overhandling of the bottle during dilution and sampling.
• Time-consuming preparation of the solution.
• Cost of the equipment used for preparation: syringes, 0.9% NaCl serum, needles, etc.
• The patients’ negative view of any injectable products that contain human or animal albumin.
2. Description of ready-to-use AboBoNT-A
- Clear, colourless liquid solution containing the same 150kDa neurotoxin and the same associated proteins as AboBoNT-A powder (Azzalure).
- Developed without any human or animal-origin dilutant (human albumin and lactose).
- Only contains plant-origin and synthetic dilutants, which maintains the toxin’s activity in a liquid preparation.
- “Green Label” eco-responsible manufacturing site, “BREEAM”.
- The bottle contains at least 125U of toxin in 0.63cc of solution. In practice, there is an upper margin of solution that allows us to estimate a total dose of more than 5%.
- Its shelf life and storage are comparable to the classic format.
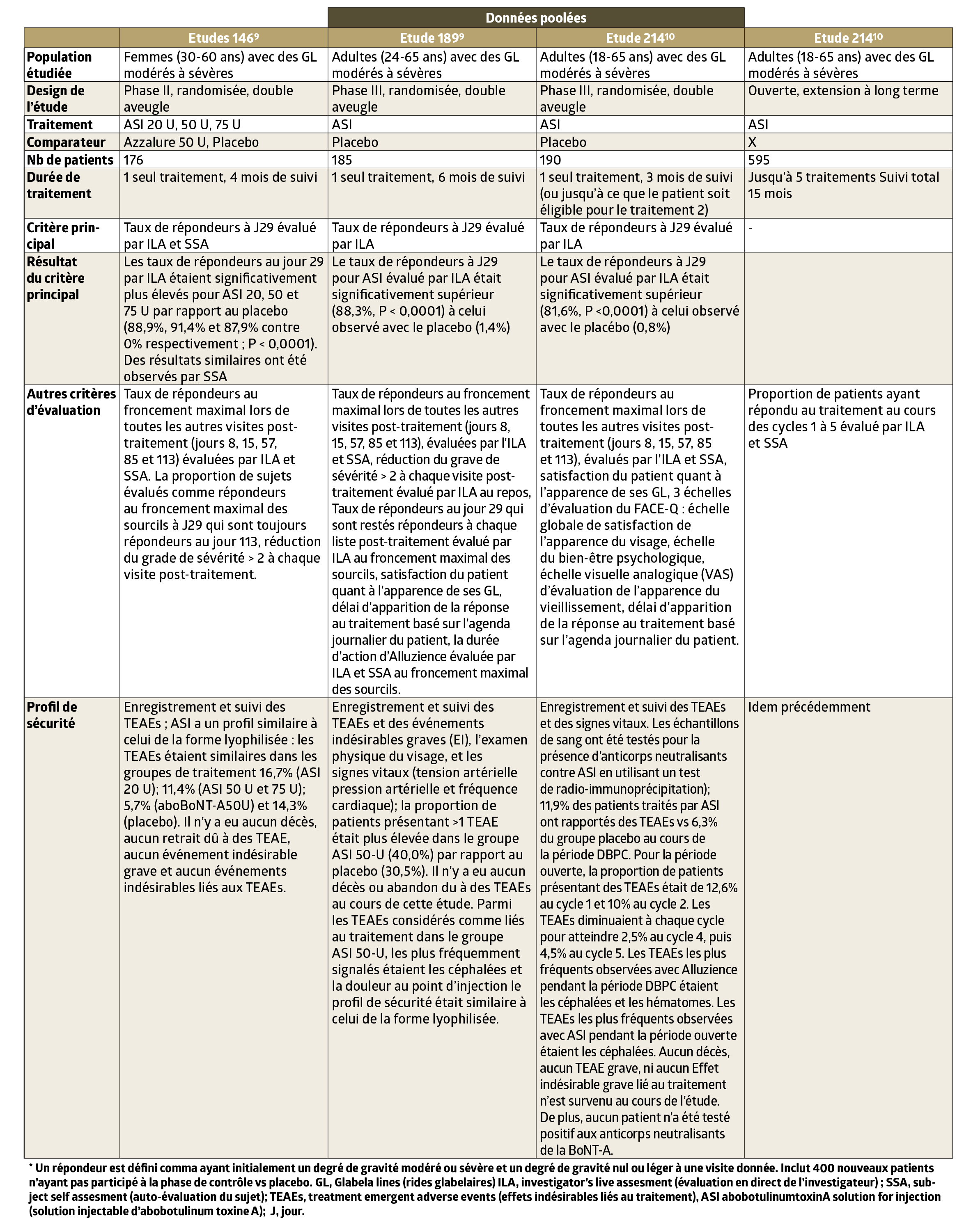
- Clinical studies have shown an increased speed of action (3 days on average, study 189), a 6-month duration of efficacy (studies189 and 214) and result reproducibility (study 214). (Fig. 3).
- High patient satisfaction (Face-Q) in these clinical studies.
- Safety and tolerance comparable to the “powder” versions.
3. Overall, what are the advantages of the liquid format?
• Precise bottle contents, without any preparation variations,which means increased safety.
• Fast clinical action.
• Duration of clinical action.
• Reproducibility of the results.
• Absence of human or animal albumin.
• Absence of neutralising antibodies found during the treatment cycles.
• Higher number of units available in the bottle compared with the “powder” format.
• Ideal for use with a 3D syringe, possible future use with ready-to-use, pre-filled syringes which would make it even easier, keeping the classic bottles for touch-ups and mini-doses.
Other companies are working on developing liquid forms of neurotoxins, which shows how much attention is being paid to innovation in this field.
 By the Doctor Philippe Kestemont
By the Doctor Philippe Kestemont
Aesthetic Head and Neck Surgeon
Doctor of Medicine in 1995 Medical Faculty of Nice, France; Graduate of the “DESC” in Head and Neck Surgery in 1997; Inter Academic Diploma in Aesthetic Face Surgery in 2001.
Associate teacher at the Medical Faculty of Nice.
Scientific Director of VISAGE, AMWC, MONACO.
Co-Director of the CADAVER COURSE, IMCAS, PARIS, FRANCE. Member of the European Academy of Facial Plastic Surgery. Member of the French Society of Facial Plastic and Aesthetic Surgery. Member of the SAMCEP Society.
More information on docteurkestemont.com