This French biotech company has provided guidance for laboratories that want to use hyaluronic acid in their products for over 30 years.
It was in France, more specifically in Ille-et-Vilaine, that the headquarters of HTL Biotechnology were set up, and the company is now established in three continents. Founded in 1992, it has a neuromodulator production site in the United States and several branches in Asia. HTL Biotechnology recently strengthened its position as a global industry leader by acquiring, through its American branch, HTL Biotechnology Innovation Inc, a platform of recombinant proteins, whose most advanced product is type III recombinant human collagen (rhCOL3), which was developed in a leading scientific research centre.
The company today employs 300 people, 18% of whom focus on R&D. Its turnover reached 100 million euros, with over 100 million euros invested between 2022 and 2027, while its production capacity was doubled in two years. HTL Biotechnology offers a unique portfolio of pharmaceutical-grade biopolymers, primarily used in aesthetic medicine fillers, but not just in this field. Hyaluronic acid is also used in ophthalmology, when producing eyedrops to soothe dry eye syndrome, in cataract surgery to protect the tissues and make the operation easier, and in rheumatology due to its viscosupplementation properties, to relieve the pain caused by arthritis.

Safety and quality
A pioneer in its field, the company stands out for producing its hyaluronic acid in fibre form, thanks to a unique process that combines precise manual skill with latest-generation technologies and equipment. Even though it is able to tailor production for every one of its clients by incorporating their different constraints, the company adheres to the most stringent international standards, namely the GMP standard (Good Manufacturing Process). The company also undergoes regular checks, with dozens of customer-led audits every year, as well as inspections carried out by major product health and safety authorities worldwide: FDA (United States), ANSM (France), as well as Korean and Japanese authorities.
Expertise in biopolymers that goes beyond hyaluronic acid

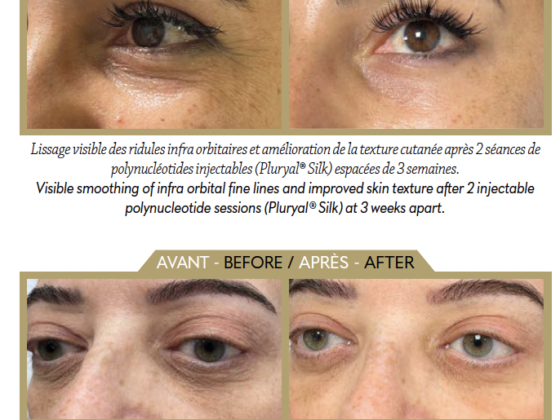
HTL’s portfolio also includes polynucleotides and collagen. Extracted in a sustainable way from salmon milt then highly purified, the polynucleotides have proven efficacy when used for biomedical and aesthetic indications, including repairing skin damage, stimulating and renewing the cells, and regulating pigmentation to improve the skin quality. Type III recombinant human collagen is currently under development, but its properties could help rebuild and protect the dermal structure to promote regeneration and improve elasticity and firmness.
The company is also carrying out research in the field of drug delivery, namely in ophthalmology, where it is being studied as a targeting vector with the aim of improving the observance and efficacy of ophthalmological treatments. It is also being studied in oncology, where it could allow for the targeted delivery of anti-cancer drugs. Finally, an interesting lead is being followed to associate biopolymers from regenerative medicine with skincare, in combinations that would create an effective synergy.
More information: htlbiotech.com