By Dr Adrián Gaspar
mesoestetic® offers two filler products containing cross-linked hyaluronic acid to improve vulvar ptosis and vulvar-vaginal atrophy or atrophic vaginitis.
 A drop in oestrogen levels leads to histological changes in the female genitourinary system that affect its function, which can lead to vulvar ptosis and the genitourinary syndrome of menopause (GSM). Despite their high prevalence and the effects these have on a woman’s quality of life, these “taboo” issues are still relatively neglected by specialists.
A drop in oestrogen levels leads to histological changes in the female genitourinary system that affect its function, which can lead to vulvar ptosis and the genitourinary syndrome of menopause (GSM). Despite their high prevalence and the effects these have on a woman’s quality of life, these “taboo” issues are still relatively neglected by specialists.
“I see more and more women in my practice who want not only to improve the appearance of their intimate area, but also to recover their sexual health and wellbeing. The menopause is the main reason behind these histological changes in the genitourinary system, but there are also other causes such as pregnancy, childbirth, weight fluctuations, medical treatments such as radiotherapy or chemotherapy, tight clothing, etc.” – Dr. Gaspar comments.
A loss of volume in the outer labia of the intimate area leads to aesthetic imperfection and, on a functional level, the overex-posure of the inner labia, which leads to excessive friction and complications during intercourse. This all has a negative impact on the patient’s sexual and psycho-emotional health. Hyaluronic acid is a molecule with a high hygroscopic capacity, meaning that its administration stimulates water retention in the mucous membranes, facilitating cell transport and trophic function, the development of new blood vessels, and collagen and elastin synthesis.
mesoestetic® have developed two products that fulfil two different requirements:
- mesofiller® sensitive (20 mg/ml) is a cross-linked hyaluronic acid gel designed to treat vaginal atrophy, dryness, dyspareunia and sexual dysfunctions. It improves the functionality of the vaginal mucous membrane, promotes the regeneration of the atrophic tissues, and increases sensitivity in the erogenous zones.
- mesofiller® sensitive plus (25 mg/ml) is designed to treat vulvar ptosis and hypertrophy in the external genital region. It fills and restores the volume of the tissues in the outer labia to preserve their protective function and improve their appearance.
Clinical results
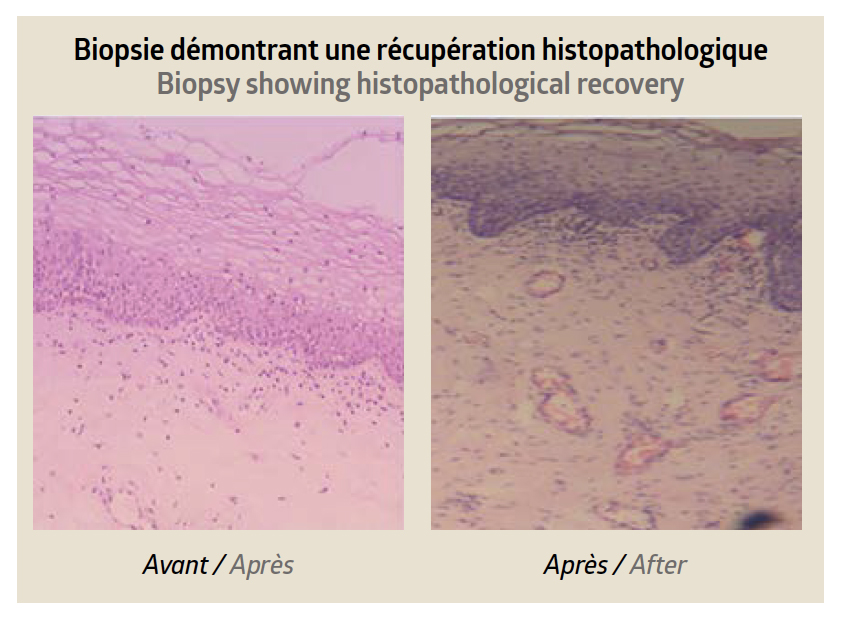 Two studies, each involving 40 patients, were carried out to evaluate the safety and efficacy of treating the intimate area with these two products containing cross-linked HA. In the first study, vulvar-vaginal atrophy or atrophic vaginitis was treated by administering 1ml of mesofiller® sensitive (20 mg/ml) by intramucosal injection into the vagina. The areas treated were the vaginal vestibule, the vaginal opening, the vaginal walls, the clitoris and the G spot. Three monitoring sessions were carried out: before treatment, after 30 days and after 60 days. In the second study, vulvar ptosis was treated by administering 1ml of mesofiller® sensitive plus (25 mg/ml) under each labia majora using a 25G x 2” cannula. Photographic evidence and the GAIS and VAS scales were used to evaluate the results after 30, 60 and 90 days.
Two studies, each involving 40 patients, were carried out to evaluate the safety and efficacy of treating the intimate area with these two products containing cross-linked HA. In the first study, vulvar-vaginal atrophy or atrophic vaginitis was treated by administering 1ml of mesofiller® sensitive (20 mg/ml) by intramucosal injection into the vagina. The areas treated were the vaginal vestibule, the vaginal opening, the vaginal walls, the clitoris and the G spot. Three monitoring sessions were carried out: before treatment, after 30 days and after 60 days. In the second study, vulvar ptosis was treated by administering 1ml of mesofiller® sensitive plus (25 mg/ml) under each labia majora using a 25G x 2” cannula. Photographic evidence and the GAIS and VAS scales were used to evaluate the results after 30, 60 and 90 days.
The results were statistically signifi cant compared with pretreatment (90% improvement on the VAS scale; p<0.0001). The results showed statistically significant results in the various clinical parameters regarding the physiology of the female intimate area. This research showed that mesofiller® sensitive and mesofiller® sensitive plus can be indicated as regenerative, functional and aesthetic treatments for vulvar-vaginal atrophy/atrophic vaginitis and vulvar ptosis, in order to reduce discomfort and improve the patients’ quality of life. Furthermore, the treatment offers many advantages compared with other methods: it is a safe, quick and minimally-invasive method that requires minimal preparation.
 Dr Adrian Gaspar: Gynaecology and obstetrics specialist and cosmetic gynaecologist. Professor of the photomedicine department in the faculty of medicine at Mendoza University, Argentina, and owner of the Espacio Gaspar centre for gynaecology, anti-aging medicine and bioregenerative medicine in Mendoza, Argentina.
Dr Adrian Gaspar: Gynaecology and obstetrics specialist and cosmetic gynaecologist. Professor of the photomedicine department in the faculty of medicine at Mendoza University, Argentina, and owner of the Espacio Gaspar centre for gynaecology, anti-aging medicine and bioregenerative medicine in Mendoza, Argentina.
More informations: dradriangaspar.ar