Dr Jean-François Bezot et Dr Claude Dalle
Telomeres are the ends of the chromosomes in eukaryote cells (imagine the end of a shoelace). They are non-coding DNA for a specific piece of information, but they are involved in chromosome stability and in the tissue aging process.
The telomeres are covered with proteins, called the shelterin complex, which acts as a kind of “bodyguard”. The shelterin complex is made up of six proteins, one of which – POT1, or Pro-tection of Telomeres protein 1 – is a kind of switch that controls access to the 3’ overhang through somatic telomerase. Telomerase is an enzyme that promotes the lengthening of the telomeres by adding TTAGG sequences to their extremities. It is active in the stem cells, but its expression is weak or even absent in somatic cells, i.e., the vast majority of human cells. When there is abundant telomerase, the telomeres are in good condition and the cells can continue to divide.
 When it is lacking (due to a genetic disorder, unhealthy lifestyle or for other reasons), the telomeres quickly shorten, and the cells stop dividing and soon enter senescence. As we age, our cells’ telomerase activity slows down and our telomeres shorten.
When it is lacking (due to a genetic disorder, unhealthy lifestyle or for other reasons), the telomeres quickly shorten, and the cells stop dividing and soon enter senescence. As we age, our cells’ telomerase activity slows down and our telomeres shorten.
Recent studies have shown that short telomeres are associated with illnesses connected with degeneration and a shorter life expectancy.
In order to stop and slow down these biological clocks, anti-aging doctors prescribe telomerase activators. These are new, cutting-edge medical treatments that have been discovered or developed over the last few years to reverse the aging process. This is a major advance. Two main types of telomerase activator currently available on the market are astralagus extract and peptides. We are delighted to welcome a third generation, which ties in with a more systemic approach thanks to a hugely promising product. On a biological level, the main factors that can affect the length of the telomeres are inflammation, infections, scarring and oxidative stress; there are therefore links between the different nutrients, the cornerstone of which is the telomere.
The impact of a number of vitamins (A, E, C) on oxidative stress, inflammation and proliferation (vitamin D), even epigenetics (vitamin B9) and DNA repair (vitamin B3), and therefore on telomere attrition, makes profi ling them an essential step. The same goes for trace elements and minerals (zinc, selenium, magnesium, etc.) and W3 polyunsaturated fatty acids. An oxi-dative stress assessment is also vital.
 To slow down cognitive decline, we must protect our neurones and the length of our telomeres by monitoring our levels of protective antioxidants and the state of our oxidative lesions (oxidised LDL, lipid peroxides) with an annual oxidative stress assessment. Nutritional and/or micro nutritional advice will be prescribed once these biological tests have been studied, in order to check that the therapies will be effective and that there is no iatrogenic risk. The patient can thus gain an additional 10 to 20%, while respecting their physiology. Some biological examinations allow us to assess the length of the telomeres. The results are expressed as a telomeric index, which is then compared to the results taken from a test population from the same age range. The telomeric index is determined by analysing the DNA contained in the nucleated cells circulating in the blood.
To slow down cognitive decline, we must protect our neurones and the length of our telomeres by monitoring our levels of protective antioxidants and the state of our oxidative lesions (oxidised LDL, lipid peroxides) with an annual oxidative stress assessment. Nutritional and/or micro nutritional advice will be prescribed once these biological tests have been studied, in order to check that the therapies will be effective and that there is no iatrogenic risk. The patient can thus gain an additional 10 to 20%, while respecting their physiology. Some biological examinations allow us to assess the length of the telomeres. The results are expressed as a telomeric index, which is then compared to the results taken from a test population from the same age range. The telomeric index is determined by analysing the DNA contained in the nucleated cells circulating in the blood.
This index reflects the average length of the telomeres and it changes with the age of the analysed cells. Consequently, a high telomeric index signals the presence of “young” cells, whereas a low telomeric index suggests “aging” cells. An indicator of cellular aging, the telomeric index reveals our biological age, as opposed to our chronological age.
The telomeric index opens the door to a method that evaluates therapeutic activity. This allows us to approve the use of micro nutritional supplements and set their dosage. Monitoring and improving the telomeric index is part of a longterm P4 Medicine© approach (Personalised, Predictive, Preventative and, above all, Participative), one of the foundations of anti-aging medicine.
Before you start taking a telomerase activator, you must have your telomeric index measured and have it checked again after 6 and 12 months of treatment to see if the average length of the telomeres has stayed the same or has increased (without treatment, they should get shorter every year), and if the patient’s number of short telomeres has reduced. A long-term study (2020-2021) about the new, third-generation product is currently being evaluated. (Cerba/Bodypur Telomere + trial). Note that, when it is too high, it can really pose a risk of cancer because we can overstimulate the stem cells, of which some are senescent with age in our body, and therefore increase the risk of cancer.
When we are able to get rid of our senescent cells, it will be fantastic!
Proper monitoring is therefore vital…and it is the short telomeres that bring about mutations. To combat senescent cells, we almost always use a product that contains quercetin, an antioxidant that is found in apples, for example.
Antioxidants can help lengthen our telomeres but so can some vitamins; the most important one is vitamin D, and recent studies have shown that its defi ciency is connected with the shortening of the telomeres.
Other vitamins are at the top of the pyramid: vitamin B9 (folate) and B12. Their pivotal role in methylation highlights the influence epigenetics has on protecting the telomeres, which is combined with that of methylation.
The most often-studied epigenetic marker in aging is DNA methylation. We know that there is a natural downward spiral that occurs as we age, just like there is a natural downward spiral in terms of our central biological clock. Yet we can combine measuring the telomeres with measuring the biological clock. This way, we get a broad view of the biological and chronological age. These two measurements have enabled us to identify certain determining factors, which modern medicine allows us to manage:
- Sleep is vital for preventing the telomeres from shortening and maintaining the biological clock.
- Night shifts are well-known for deregulating our bodies.
- Melatonin is a synchroniser and helps stabilise the telomeres.
- Most cell repair is carried out during the night.
- Physical, inflammatory and psychological stress is also at the heart of this machinery and its potential wear and tear.
- A lack of oestrogen, like we see after the menopause, shortens the telomeres.
- As does obesity.
- When stimulated, oxytocin slows down telomere wear and tear, hence the importance of social interaction and pleasurable pursuits (eating, listening to music, enjoying beautiful scenery, etc.
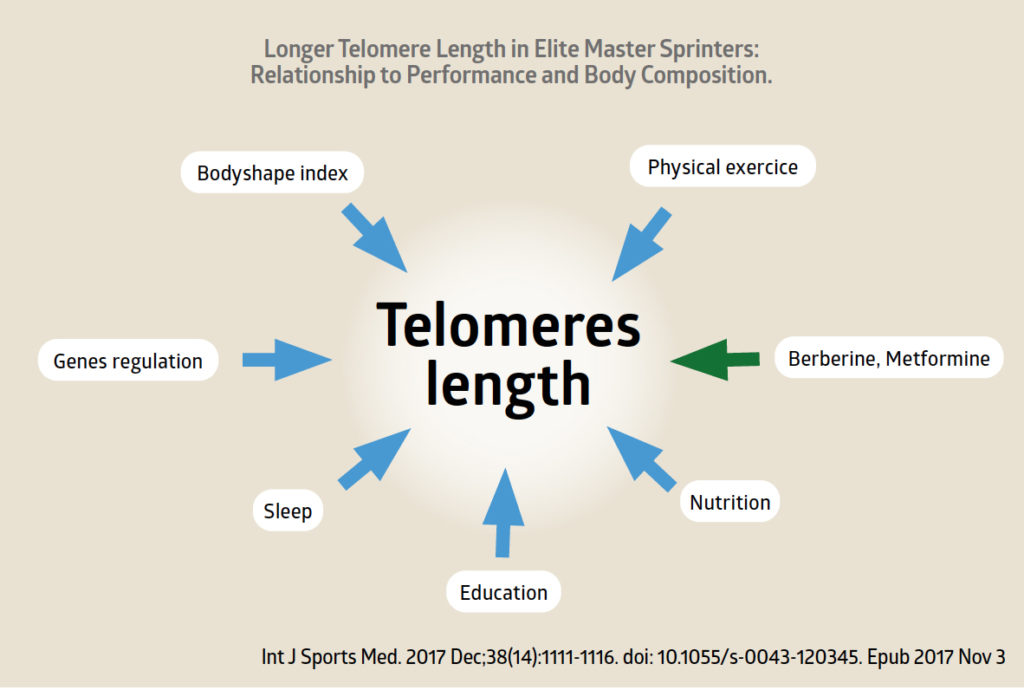
- Moderate physical exercise might be added to improve our telomeric index, but excessive exercise seems to have a negative effect. Five hours per week after age 50 is a good amount.
- Key molecules have come to light, like metformin, that stabilise our telomeric index. What is more, melatonin and metformin have been found to have cancer-fighting properties. Berberine might also be included in this group.
- Astragalus and gingko biloba, too.
- And, of course, omega 3s, including DHA.
- However, any prolonged glycation, due to excess sugar or pollution (benzopyrene, acrylamide, etc.) has a negative effect on our telomeres.
- Some metals, such as zinc, have a key function; this metal is present in our DNA, it is an essential cofactor of SOD in the fight against excess oxidation. As is selenium, a cofactor of glutathione, without which methylation starts to slide.
- We must not forget renal function, which is at the helm of this system (homocysteine, uricemia).
- Sirtuins, enzymes that regulate our longevity, keep the telomeres stable. They are activated by fasting, calorie restriction, and certain molecules that imitate fasting such as berberine or metformin.
The stability of the genome is at the heart of aging, homeostasis is one of the key words. Luckily, we can change the length of our telomeres. Thanks to a set of twins – one of whom stayed on Earth and the other who travelled to the international space station – we were able to see that the twin who went into space’s telomeres lengthened, but this phenomenon stopped when they came back to Earth. So, it seems we age more slowly in space. Telomeres have turned out to be vital controllers in our biological arsenal. Once they have been assessed, it is down to us to react and adapt in order to fi nd ways to optimise our health. It is lovely to see our index improve, to see our efforts rewarded after just a few months. We are well aware of the levers and we can therefore combine astralagus, suitable vitamins, and reputed molecules. Nature gives us the keys, biology and medicine the locks. It is vital that we monitor the markers of tissue inflammation before and after any courses of treatment. Mutations can cause cancer and short telomeres lead to mutations.
Let us not forget that the Greek “Telos”, from the gospels, means eternity not immortality.
 Doctor Jean-François Bezot Medical biologist. Pharmaceutical doctor, Paris Pharmaceutical Faculty. Former house pharmacist in the Paris Hospitals. Has specialised in anti-aging biology and functional proteomics since 1988. Vice-president of the French Society for Anti-Aging Medicine. International conference speaker.
Doctor Jean-François Bezot Medical biologist. Pharmaceutical doctor, Paris Pharmaceutical Faculty. Former house pharmacist in the Paris Hospitals. Has specialised in anti-aging biology and functional proteomics since 1988. Vice-president of the French Society for Anti-Aging Medicine. International conference speaker.
More informations: biopredix.com
 Doctor Claude Dalle Degree in acupuncture, mesotherapy, Eriksonian hypnotherapy. Speaker at international conferences, course leader at the Faculty of Medicine in Paris. Scientifi c director of congresses.
Doctor Claude Dalle Degree in acupuncture, mesotherapy, Eriksonian hypnotherapy. Speaker at international conferences, course leader at the Faculty of Medicine in Paris. Scientifi c director of congresses.
More informations: drclaudedalle.com